Full Automatic Rotary Pouch Maker with Internal Printer and Labeller Model: 50GM 5LCS1PVIP - SHORT CODE: 50GM Rotary Pouch making machine with integrated printer and Labeler for the automatic production of flat, gusseted and Tyvek® pouches in hospitals and the medical industry It meets the requirements of the World Health Organization (WHO) guideline as well as the current guideline of the World Federation of Hospital Sterilization Sciences (WFHSS). It provides verifiable sealing action according to EN ISO 11607-2 and international guide ISO/TS 16775. It documents this in writing with the Validation Report at each first use or when requested.
Full Automatic Rotary Pouch Maker with Internal Printer and Labeller 50GM 5LCS1PVIP - SHORT CODE: 50GM
Rotary Pouch making machine with integrated printer and Labeler for the automatic production of flat, gusseted and Tyvek® pouches in hospitals and the medical industry
With Single Line Printer 222-300-050_50GM 5LCS1PVIP
With Double Line Printer 222-300-052_52GM 5LCS2PVIP
It meets the requirements of the World Health Organization (WHO) guideline as well as the current guideline of the World Federation of Hospital Sterilization Sciences (WFHSS).
It provides verifiable sealing action according to EN ISO 11607-2 and international guide ISO/TS 16775. It documents this in writing with the Validation Report at each first use or when requested.
- Multi-Roll-Processing®: Capacity to process 8 rolls of 5cm or different widths up to 43cm at the same time.
- Ability to work in 5 different modes; 1- Cutting, 2- Cutting and Sealing, 3- Cutting and Sealing and Writing, 4- Sealing, 5- Sealing and Writing
- Fast-Sealing®: Pouch processing capacity at 10 / 12 / 14 or 16 m/min.
- Production rate of 5000 pouches per hour: Reduces personnel costs by 70%
- Print-To-CutSide®: Print-To-CutSide®: The only device in the world that can write data on both sides of the pouche
- Computer-based: 7” color interactive display and multilingual
- Login: No password or password protected login for users
- Write-Matic®: Ability to write long texts in a narrow pouch
- Auto-Slider®: Automatic mode switching without removing the rolls from the device and without changing the settings
- No-Ribbon®: Prevent unnecessary operation of the printer module when there is no print cartridge in the device
- Sealing type and width: MultiLine closure, 14mm
- 9999 product/set memory: Expiry Date, Pouch width and barcode recording for each product or set separately
- Stand-By: Stand-By: If no pouch is supplied to the device, the motor stops after 30 seconds, mechanical protection
- Sleep Mode®: If the device is not used for a long time, after the desired (adjustable) time, shutting down the entire system including the heaters, energy saving*.
- Soft-Start®: Soft-Start®: Slow start for heavy packages
- Personnel safety: The Emergency Stop Button on the device immediately cuts off all energy.
- Device safety: The pouch jammed in the device can be removed immediately with the Emergency Back, Emergency Stop and Emergency Forward buttons on the screen or with the mechanical key.
- Customized Teflon-Coating®: Pouches that melt in the device cannot stick to the heaters. In this way, the work does not stop, spare parts, service, maintenance are not required.
- Cutting Process: Self-sharpening round blade
- Free software update from the Internet: Wi-Fi and/or Wired (Ethernet cable)
- Integrated internal thermal printer; Self-adhesive labels identify and hold manufactured pouches together
- Connection: Connection to QR code reader, Keyboard, Mouse and TV (Optional)
- Easy serviceability: easy access to parts thanks to modular device structure
- Maintenance time: 1 time per year
- Warranty: 2 Years
- Lifespan: >15 years
* The “Sleep-Mode®” (Energy Saving System) was applied to the GÜNDEM sealers for the first time in the world in 2009.
Under the "Related Products" heading below, you can see the accessories that can be used with this product, for detailed technical data; you can download the brochure, technical specifications (Membership may require) and quality certificates, etc. of that product from the "Files" tab above. You can watch the operation of the product from the "Videos" tab, if available.
Brochure: 50-52GM-GM5LCS1PV Cut-Seal-1 Line Printing+Labeling Device
222-300-050-1ML_50GM-GM5LCS1PV-20240708-Rpc
ISO 10002-2018-Customer Satisfaction and Complaints Management System
ISO 10002-2018-20250712-Müşteri Memnuniyeti ve Şikayetleri Yönetim Sistemi
ISO 9001 : 2015
ISO 9001-2015-20250712-Kalite Yönetim Sistemi
Our manufactures - Presentation File
200-000-000-8ML_SunumPresentationSealers-Rev2.5-20240127 .pdf
Catalogue-Sealing Machines
200-000-000-0En_GeneralCatalog-Rev2.0-20231113
All Sealer Accessories and Other Products
200-000-000-1En_Accessories-20231113
Related products
Roller Conveyor Extra Large – for Automatic Rotary Pouch Maker Sealers A045 GM140RTXL – SHORT CODE: A045
Conveyor helps the user in the packaging process and prevents package damage.
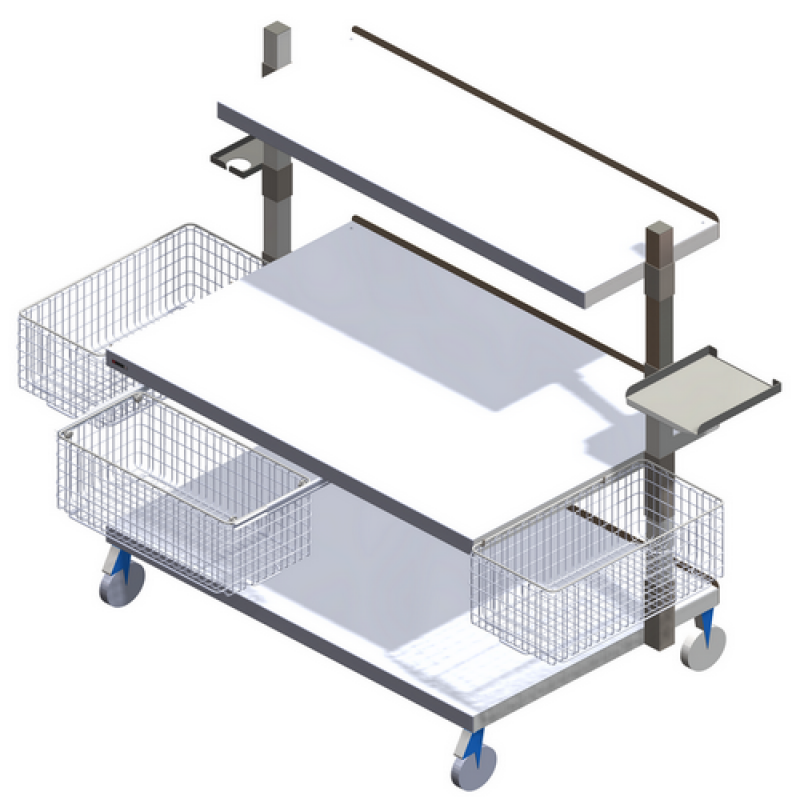
Extra-Wide Mobile Workstation for Full Automatic Rotary Pouch Makers A105 GM1215WST – SHORT CODE: A105
Provides a workstation solution that meets all the requirements of mobile and modular workstations,...
Thermal Label Roll for Automatic Pouch Makers A160 GM160THL – SHORT CODE: A160
It acts as a scattering, binding and defining the produced pouches.
Thermal Paper Roll for Automatic Pouch Makers A155 GM155THP – SHORT CODE: A155
It acts as a scattering, binding and defining the produced pouches.
Seal Check Test for Sealing Quality Testing of All Sealers Standard A165 GM165SCS – SHORT CODE: A165
It is a test that the devices work in accordance with EN-ISO 11607-2 standard and factory settings.
Seal Check Test for Sealing Quality Testing of All Sealers for H2O2/Plasma A170 GM170SCT– SHORT CODE: A170
It is a test that the devices work in accordance with EN-ISO 11607-2 standard and factory settings.
Ink Ribbon for Sealers with Printer A175 GM175IRB – SHORT CODE: A175
It allows data to be written on the sealed pouches
© 2025. All Rights Reserved. | General Data Protection Regulation | Cookie Policy | Cookie Preferences Management